HEAT
HEAT
Heat is a form of energy. It is produced only by the conversion of one
of the other forms of energy. Heat may also be defined as the total kinetic
energy of the molecules of any substance.
Some forms of energy which can be converted into heat energy are
as follows:
(1) Mechanical Energy. This includes all methods of producing increased
motion of molecules such as friction, impact of bodies, or compression
of gases.
(2) Electrical Energy. Electrical energy is converted to heat energy
when an electric current flows through any form of resistance. This might
be an electric iron, electric light, or an electric blanket.
(3) Chemical Energy. Most forms of chemical reaction convert stored
potential energy into heat. Some examples are the explosive effects of
gunpowder, the burning of oil or wood, and the combining of oxygen and
grease.
(4) Radiant Energy. Electromagnetic waves of certain frequencies produce
heat when they are absorbed by the bodies they strike. Included are X-rays,
light rays, and infrared rays.
(5) Nuclear Energy. Energy stored in the nucleus of atoms is released
during the process of nuclear fission in a nuclear reactor or atomic explosion.
(6) The Sun. All heat energy can be directly or indirectly traced to
the nuclear reactions occurring in the sun.
Mechanical Equivalent of Heat
When a gas is compressed, work is done and the gas becomes warm or hot.
Conversely, when a gas under high pressure is allowed to expand, the expanding
gas becomes cool. In the first case, work was converted into energy in
the form of heat; in the second case heat energy was expended. Since heat
is given off or absorbed, there must be a relationship between heat energy
and work. Also, when two surfaces are rubbed together, the friction develops
heat. However, work was required to cause the heat, and by experimentation,
it has been shown that the work required and the amount of heat produced
by friction are proportional. Thus, heat can be regarded as a form of energy.
According to this theory of heat as a form of energy, the molecules,
atoms, and electrons in all bodies are in a continual state of motion.
In a hot body, these small particles possess relatively large amounts of
kinetic energy, but in cooler bodies they have less. Because the small
particles are given motion, and hence kinetic energy, work must be done
to slide one body over the other. Mechanical energy apparently is transformed,
and what we know as heat is really kinetic energy of the small molecular
subdivisions of matter.
Two different units are used to express quantities of heat energy. They
are the calorie and the British thermal unit. One calorie is equal to the
amount of heat required to change the temperature of 1 gram of water 1
degree centigrade. This term "calorie" (spelled with a small c) is 1/1,000
of the Calorie (spelled with a capital C) used in the measurement of heat
producing or energy producing value in foods. One Btu (British thermal
unit) is defined as the amount of heat required to change the temperature
of 1 pound of water 1 degree Fahrenheit. The calorie and the gram are seldom
used in discussing aviation maintenance. The Btu, however, is commonly
referred to in discussions of engine thermal efficiencies and the heat
content of aviation fuel.
A device known as the calorimeter is used to measure quantities of heat
energy. For example, it may be used to determine the quantity of heat energy
available in 1 pound of aviation gasoline. A given weight of the fuel is
burned in the calorimeter, and the heat energy is absorbed by a large quantity
of water. From the weight of the water and the increase in its temperature,
it is possible to compute the heat yield of the fuel. A definite relationship
exists between heat and mechanical energy. This relationship has been established
and verified by many experiments which show that:
One Btu = 778 ft-lbs
Thus, if the 1 pound sample of the fuel mentioned above were found to
yield 20,000 Btu, it would be the equivalent of 20,000 Btu x 778 ft-lbs/Btu
or 15,560,000 ft-lbs of mechanical energy.
Unfortunately no heat engine is capable of transforming all of the available
heat energy in the fuel it burns into mechanical energy. A large portion
of this energy is wasted through heat losses and operational friction.
Methods of Heat Transfer
There are three methods by which heat is transferred from one location
to another or from one substance to another. These three methods are conduction,
convection, and radiation.
| Conduction
Everyone knows from experience that the metal handle of a heated pan
can burn the hand. A plastic or wood handle, however, remains relatively
cool even though it is in direct contact with the pan. The metal transmits
the heat more easily than the wood because it is a better conductor of
heat. Different materials conduct heat at different rates. Some metals
are much better conductors of heat than others. Aluminum and copper are
used in pots and pans because they conduct heat very rapidly. Woods and
plastics are used for handles because they conduct heat very slowly.
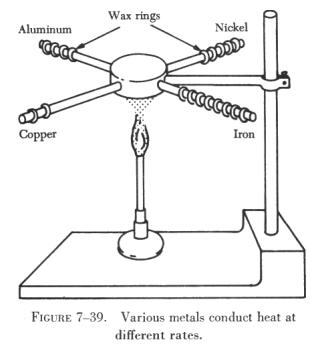
Figure 7-39 illustrates the different rates of conduction of various
metals. Four rods of different metals have several wax rings handing on
them. One flame is used to heat one end of each rod simultaneously. The
rings melt and drop off the copper rod first, then from the aluminum rod,
then from the nickel rod, and last from the iron rod. This example shows
that among the four metals used, copper is the best conductor of heat and
iron is the poorest. |
|
 |
Liquids are poorer conductors of heat than metals. Notice that the
ice in the test tube shown in figure 7-40 is not melting rapidly even though
the water at the top is boiling. The water conducts heat so poorly that
not enough heat reaches the ice to melt it.
Gases are even poorer conductors of heat than liquids. It is possible
to stand quite close to a stove without being burned because air is such
a poor conductor. Since conduction is a process whereby the increase in
molecular energy is passed along by actual contact, gases are poor conductors.
At the point of application of the heat source the molecules become
violently agitated. These molecules strike adjacent molecules causing them
to become agitated. This process continues until the heat energy is distributed
evenly throughout the substance. Because molecules are farther apart in
gases than in solids, the gases are much poorer conductors of heat. |
Materials that are poor conductors are used to prevent the transfer
of heat and are called heat insulators. A wooden handle on a pot or a soldering
iron serves as a heat insulator. Certain materials such as finely spun
glass or asbestos are particularly poor heat conductors. These materials
are therefore used for many types of insulation.
| Convection
Convection is the process by which heat is transferred by movement of
a heated fluid (gas or liquid). For example, an electronic tube will, when
heated, become increasingly hotter until the air surrounding it begins
to move. The motion of the air is upward. This upward motion of the heated
air carries the heat away from the hot tube by convection. Transfer of
heat by convection may be hastened by using a ventilating fan to move the
air surrounding a hot object. The rate of cooling of a hot vacuum tube
can be increased if it is provided with copper fins that conduct heat away
from the hot tube. The fins provide large surfaces against which cool air
can be blown.
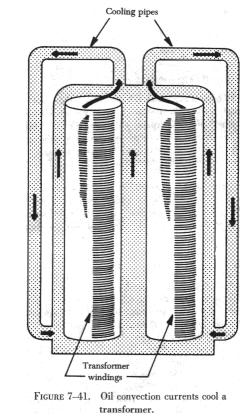
A convection process may take place in a liquid as well as in a gas.
Figure 7-41 shows a transformer in an oil bath. The hot oil is less dense
(has less weight per unit volume) and rises, while the cool oil falls,
is heated, and rises in turn.
When the circulation of gas or liquid is not rapid enough to remove
sufficient heat, fans or pumps are used to accelerate the motion of the
cooling material. In some installations, pumps are used to circulate water
or oil to help cool large equipment. In airborne installations electric
fans and blowers are used to aid convection. |
|
Radiation
Conduction and convection cannot wholly account for some of the phenomena
associated with heat transfer. For example, the heat one feels when sitting
in front of an open fire cannot be transferred by convection because the
air currents are moving toward the fire. It cannot be transferred through
conduction because the conductivity of the air is very small, and the cooler
currents of air moving toward the fire would more than overcome the transfer
of heat outward. Therefore, there must be some way for heat to travel across
space other than by conduction and convection.
The existence of another process of heat transfer is still more evident
when the heat from the sun is considered. Since conduction and convection
take place only through some medium, such as a gas or a liquid, heat from
the sun must reach the earth by another method, since space is an almost
perfect vacuum. Radiation is the name given to this third method of heat
transference.
The term "radiation" refers to the continual emission of energy from
the surface of all bodies. This energy is known as radiant energy. It is
in the form of electromagnetic waves, radio waves, or X-rays, which are
all alike except for a difference in wave lengths. These waves travel at
the velocity of light and are transmitted through a vacuum more easily
than through air because air absorbs some of them. Most forms of energy
can be traced back to the energy of sunlight. Sunlight is a form of radiant
heat energy which travels through space to reach the earth. These electromagnetic
heat waves are absorbed when they come in contact with nontransparent bodies.
The result is that the motion of the molecules in the body is increased
as indicated by an increase in the temperature of the body.
The differences between conduction, convection, and radiation may now
be considered. First, although conduction and convection are extremely
slow, radiation takes place with the speed of light. This fact is evident
at the time of an eclipse of the sun when the shutting off of the heat
from the sun takes place at the same time as the shutting off of the light.
Second, radiant heat may pass through a medium without heating it. For
example, the air inside a greenhouse may be much warmer than the glass
through which the sun's rays pass. Third, although conducted or convected
heat may travel in roundabout routes, radiant heat always travels in a
straight line. For example, radiation can be cut off with a screen placed
between the source of heat and the body to be protected.
The sun, a fire, and an electric light bulb all radiate energy, but
a body need not glow to give off heat. A kettle of hot water or a hot soldering
iron radiates heat. If the surface is polished or light in color, less
heat is radiated. Bodies which do not reflect are good radiators and good
absorbers, and bodies that reflect are poor radiators and poor absorbers.
For this reason white clothing is worn in the summer season.
A practical example of the control of loss of heat is the thermos bottle.
The flask itself is made of two walls of glass separated by a vacuum. The
vacuum prevents the loss of heat by conduction and convection, and a silver
coating on the walls prevents the loss of heat by radiation.
Specific Heat
One important way in which substances differ is in the requirement of
different quantities of heat to produce the same temperature change in
a given mass of the substance. Each substance requires a quantity of heat,
called its specific heat capacity, to increase the temperature of a unit
of its mass 1° C. The specific heat of a substance is the ratio of
its specific heat capacity to the specific heat capacity of water. Specific
heat is expressed as a number which, because it is a ratio, has no units
and applies to both the English and the metric systems.
It is fortunate that water has a high specific heat capacity. The larger
bodies of water on the earth keep the air and solid matter on or near the
surface of the earth at a fairly constant temperature. A great quantity
of heat is required to change the temperature of a large lake or river.
Therefore, when the temperature falls below that of such bodies of water,
they give off large quantities of heat. This process keeps the atmospheric
temperature at the surface of the earth from changing rapidly.
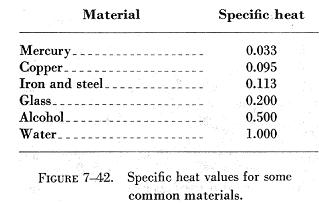
The specific heat values of some common materials are listed in figure
7-42.
Thermal Expansion
Thermal expansion takes place in solids, liquids, and gases when they
are heated. With few exceptions, solids will expand when heated and contract
when cooled. Because the molecules of solids are much closer together and
are more strongly attracted to each other, the expansion of solids when
heated is very slight in comparison to the expansion in liquids and gases.
The expansion of fluids has been discussed in the study of Boyle's law.
Thermal expansion in solids must be explained in some detail because of
its close relationship to aircraft metals and materials.
Expansion in Solids
| Solid materials expand in length, width, and thickness
when they are heated. An example of the expansion and contraction of substances
is the ball and ring, illustrated in figure 7-43. The ball and ring are
made of iron. When both are at the same temperature, the ball will barely
slip through the ring. When the ball is heated or the ring is cooled, however,
the ball cannot slip through the ring.
Experiments show that for a given change in temperature, the change
in length or volume is different for each substance. For example, a given
change in temperature causes a piece of copper to expand nearly twice as
much as a piece of glass of the same size and shape. For this reason, the
lead wires into an electronic tube cannot be made of copper but must be
made of a metal that expands at the same rate as glass. If the metal does
not expand at the same rate as the glass, the vacuum in the tube is broken
by air leaking past the wires in the glass stem. |

|
Because some substances expand more than others, it is necessary to
measure experimentally the exact rate of expansion of each one. The amount
that a unit length of any substance expands for a one degree rise in temperature
is known as the coefficient of linear expansion for that substance.
Coefficients of Expansion
To estimate the expansion of any object, such as a steel rail, it is
necessary to know three things about it; namely, its length, the rise in
temperature to which it is subjected, and its coefficient of expansion.
This relationship is expressed by the equation:
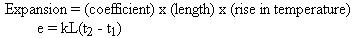
In this equation, the letter "k" represents the coefficient of expansion
for the particular substance. In some instances,

then:

| This amount, when added to the original length of the rod, makes the
rod 9.00306 feet long.
The increase in the length of the rod is relatively small; but if the
rod were placed where it could not expand freely, there would be a tremendous
force exerted due to thermal expansion. Thus, thermal expansion must be
taken into consideration when designing airframes, powerplants, or related
equipment.
Figure 7-44 contains a list of the coefficients of linear expansion
for some common substances. |
 |
 |
A practical application which uses the difference in the coefficients
of linear expansion of metals is the thermostat. This instrument consists
of an arrangement of two bars of dissimilar metal fastened together. When
the temperature changes, a bending takes place because of the unequal expansion
of the metals. Figure 7-45 shows such an instrument, made with a wooden
handle for laboratory demonstrations. Thermostats are used in overload
relays in motors, in temperature sensitive switches, and in heating systems.
|








