The aircraft gas turbine is designed to operate on a distillate fuel,
commonly called jet fuel. Jet fuels are also composed of hydrocarbons with
a little more carbon and usually a higher sulfur content than gasoline.
Inhibitors may be added to reduce corrosion and oxidation. Anti-icing additives
are also being blended to prevent fuel icing.
 |
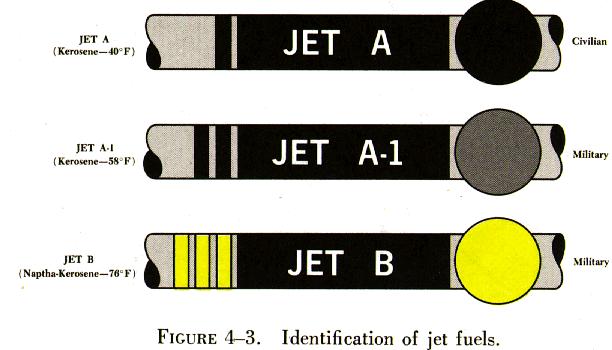
Two types of jet fuel in common use today are: (1) Kerosene grade turbine fuel, now named Jet A; and (2) a blend of gasoline and kerosene fractions, designated Jet B. There is a third type, called Jet A-1, which is made for operation at extremely low temperatures. See figure 4-3.
There is very little physical difference between Jet A (JP-5) fuel and commercial kerosene. Jet A was developed as a heavy kerosene having a higher flash point and lower freezing point than most kerosenes. It has a very low vapor pressure, so there is little loss of fuel from evaporation or boil off at higher altitudes. It contains more heat energy per gallon than does Jet B (JP-4).
Jet B is similar to Jet A. It is a blend of gasoline and kerosene fractions. Most commercial turbine engines will operate on either Jet A or Jet B fuel. However, the difference in the specific gravity of the fuels may require fuel control adjustments. Therefore, the fuels cannot always be considered interchangeable.
Both Jet A and Jet B fuels are blends of heavy distillates and tend to absorb water. The specific gravity of jet fuels, especially kerosene, is closer to water than is aviation gasoline; thus, any water introduced into the fuel, either through refueling or condensation, will take an appreciable time to settle out. At high altitudes, where low temperatures are encountered, water droplets combine with the fuel to form a frozen substance referred to as "gel." The mass of "gel" or "icing" that may be generated from moisture held in suspension in jet fuel can be much greater than in gasoline.
Volatility
One of the most important characteristics of a jet fuel is its volatility. It must, of necessity, be a compromise between several opposing factors. A highly volatile fuel is desirable to aid in starting in cold weather and to make aerial restarts easier and surer.
Low volatility is desirable to reduce the possibility of vapor lock
and to reduce fuel losses by evaporation. At normal temperatures, gasoline
in a closed container or tank can give off so much vapor that the fuel/air
mixture may be too rich to burn. Under the same conditions, the vapor given
off by Jet B fuel can be in the flammable or explosive range. Jet A fuel
has such a low volatility that at normal temperatures it gives off very
little vapor and does not form flammable or explosive fuel/air mixtures.
Figure 4-4 shows the vaporization of aviation fuels at atmospheric pressure.
 |
Identification
Because jet fuels are not dyed, there is no on sight identification for them. They range in color from a colorless liquid to a straw colored (amber) liquid, depending on age or the crude petroleum source.
Jet fuel numbers are type numbers and have no relation to the fuel's performance in the aircraft engine.