CHARACTERISTICSANDPROPERTIESOFAVIATIONGASOLINE
CHARACTERISTICS AND PROPERTIES
OF AVIATION GASOLINE
Aviation gasoline consists almost entirely of hydrocarbons, namely,
compounds consisting of hydrogen and carbon. Some impurities in the form
of sulfur and dissolved water will be present. The water cannot be avoided,
since the gasoline is exposed to moisture in the atmosphere. A small amount
of sulfur, always present in crude petroleum, is left in the process of
manufacture.
Tetraethyl lead (TEL) is added to the gasoline to improve its performance
in the engine. Organic bromides and chlorides are mixed with TEL so that
during combustion volatile lead halides will be formed. These then are
exhausted with the combustion products. TEL, if added alone, would burn
to a solid lead oxide and remain in the engine cylinder. Inhibitors are
added to gasoline to suppress the formation of substances that would be
left as solids when the gasoline evaporates.
Certain properties of the fuel affect engine performance. These properties
are volatility, the manner in which the fuel burns during the combustion
process, and the heating value of the fuel. Also important is the corrosiveness
of the gasoline as well as its tendency to form deposits in the engine
during use. These latter two factors are important because of their effect
on general cleanliness, which has a bearing on the time between engine
overhauls.
Volatility
Volatility is a measure of the tendency of a liquid substance to vaporize
under given conditions. Gasoline is a complex blend of volatile hydrocarbon
compounds that have a wide range of boiling points and vapor pressures.
It is blended in such a way that a straight chain of boiling points is
obtained. This is necessary to obtain the required starting, acceleration,
power, and fuel mixture characteristics for the engine.
If the gasoline vaporizes too readily, fuel lines may become filled
with vapor and cause decreased fuel flow. If the fuel does not vaporize
readily enough, it can result in hard starting, slow warmup, poor acceleration,
uneven fuel distribution to cylinders, and excessive crankcase dilution.
The lower grades of automobile fuel are not held within the tolerances
required for aviation gasoline and usually contain a considerable amount
of cracked gasoline, which may form excessive gum deposits. For these reasons,
automobile fuels should not be used in aircraft engines, especially air
cooled engines operating at high cylinder temperatures.
| Vapor Lock
Vaporization of gasoline in fuel lines results in a reduced supply of
gasoline to the engine. In severe cases, it may result in engine stoppage.
This phenomenon is referred to as vapor locking. A measure of a gasoline's
tendency to vapor lock is obtained from the Reid vapor pressure test. In
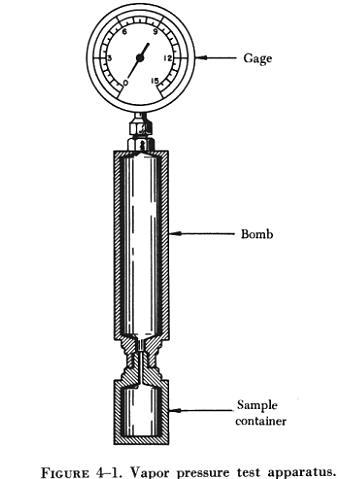
this test a sample of the fuel is sealed in a "bomb" equipped with a pressure
gauge. The apparatus (see figure 4-1) is then immersed in a constant temperature
bath and the indicated pressure is noted. The higher the corrected vapor
pressure of the sample under test, the more susceptible it is to vapor
locking. Aviation gasolines are limited to a maximum of 7 psi because of
their increased tendency to vapor lock at high altitudes.
Carburetor Icing
Carburetor icing is also related to volatility. When the fuel changes
from a liquid to a vapor state, it extracts heat from its surroundings
to make this change. The more volatile the fuel, the more rapid the heat
extraction will be. As the gasoline leaving the carburetor discharge nozzle
vaporizes, it can freeze water vapor contained in the incoming air. The
moisture freezes on the walls of the induction system, the venturi throat,
and the throttle valves. This type of ice formation restricts the fuel
and air passages of the carburetor. It causes loss of power and, if not
eliminated, eventual engine stoppage. Extreme icing conditions can make
operation of the throttle controls impossible. This icing condition is
most severe in the temperature range of 30° to 40° F outside air
temperature. |
|
Aromatic Fuels
Some fuels may contain considerable quantities of aromatic hydrocarbons,
which are added to increase the rich mixture performance rating of the
fuel. Such fuels, known as aromatic fuels, have a strong solvent and swelling
action on some types of hose and other rubber parts of the fuel system.
For this reason, aromatic resistant hose and rubber parts have been developed
for use with aromatic fuels.
Detonation
In an engine that is operating in a normal manner, the flame front traverses
the charge at a steady velocity of about 100 feet per second until the
charge is consumed. When detonation occurs, the first portion of the charge
burns in a normal manner but the last portion burns almost instantaneously,
creating an excessive momentary pressure unbalance in the combustion chamber.
This abnormal type of combustion is called detonation. This tremendous
increase in the speed of burning causes the cylinder head temperature to
rise. In severe cases, the increase in burning speed will decrease engine
efficiency and may cause structural damage to the cylinder head or piston.
During normal combustion, the expansion of the burning gases presses the
head of the piston down firmly and smoothly without excessive shock. The
increased pressure of detonation exerted in a short period of time produces
a heavy shock load to the walls of the combustion chamber and the piston
head. It is this shock to the combustion chamber that is heard as an audible
knock in an automobile engine. If other sounds could be filtered out, the
knock would be equally audible in an aircraft engine. Generally, it is
necessary to depend upon instruments to detect detonation in an aircraft
engine.
Surface Ignition
Ignition of the fuel/air mixture by hot spots or surfaces in the combustion
chamber is called surface ignition. If this occurs before the normal ignition
event, the phenomenon is referred to as preignition. When it is prevalent,
the result is power loss and engine roughness. Preignition is generally
attributed to overheating of such parts as spark plug electrodes, exhaust
valves, carbon deposits, etc. Where preignition is present, an engine may
continue to operate even though the ignition has been turned off. Present
information indicates that gasoline high in aromatic hydrocarbon content
is much more likely to cause surface ignition than fuels with a low content.
Octane and Performance Number Rating
Octane and performance numbers designate the antiknock value of the
fuel mixture in an engine cylinder. Aircraft engines of high power output
have been made possible principally as a result of blending to produce
fuels of high octane ratings. The use of such fuels has permitted increases
in compression ratio and manifold pressure, resulting in improved engine
power and efficiency. However, even the high octane fuels will detonate
under severe operating conditions and when certain engine controls are
improperly operated.
Antiknock qualities of aviation fuel are designated by grades. The higher
the grade, the more compression the fuel can stand without detonating.
For fuels that have two numbers, the first number indicates the lean mixture
rating and the second the rich mixture rating. Thus, grade 100/130 fuel
has a lean mixture rating of 100 and a rich mixture rating of 130. Two
different scales are used to designate fuel grade. For fuels below grade
100, octane numbers are used to designate grade. The octane number system
is based on a comparison of any fuel with mixtures of iso octane and normal
heptane. The octane number of a fuel is the percentage of iso octane in
the mixture that duplicates the knock characteristics of the particular
fuel being rated. Thus, grade 91 fuel has the same knock characteristics
as a blend of 91 percent iso octane and 9 percent normal heptane.
With the advent of fuels having antiknock characteristics superior to
iso octane, another scale was adopted to designate the grade of fuels above
the 100 octane number. This scale represents the performance rating of
the fuel - its knock free power available as compared with that available
with pure iso octane. It is arbitrarily assumed that 100 percent power
is obtained from iso octane alone. An engine that has a knock limited horsepower
of 1,000 with 100 octane fuel will have a knock limited horsepower of 1.3
times as much (1,300 horsepower) with 130 performance number fuel.
The grade of an aviation gasoline is no indication of its fire hazard.
Grade 91/96 gasoline is as easy to ignite as grade 115/145 and explodes
with as much force. The grade indicates only the gasoline's performance
in the aircraft's engine.
A convenient means of improving the antiknock characteristics of a fuel
is to add a knock inhibitor. Such a fluid must have a minimum of corrosive
or other undesirable qualities, and probably the best available inhibitor
in general use at present is TEL (tetraethyl lead). The few difficulties
encountered because of the corrosion tendencies of ethylized gasoline are
insignificant when compared with the results obtained from the high antiknock
value of the fuel. For most aviation fuels the addition of more than 6
ml. per gallon is not permitted. Amounts in excess of this have little
effect on the antiknock value, but increase corrosion and spark plug trouble.
There are two distinct types of corrosion caused by the use of ethyl
gasoline. The first is caused by the reaction of the lead bromide with
hot metallic surfaces, and occurs when the engine is in operation; the
second is caused by the condensed products of combustion, chiefly hydrobromic
acid, when the engine is not running.
Purity
Aviation fuels must be free of impurities that would interfere with
the operation of the engine or the units in the fuel and induction system.
Even though all precautions are observed in storing and handling gasoline,
it is not uncommon to find a small amount of water and sediment in an aircraft
fuel system. A small amount of such contamination is usually retained in
the strainers in the fuel system. Generally, this is not considered a source
of great danger, provided the strainers are drained and cleaned at frequent
intervals. However, the water can present a serious problem because it
settles to the bottom of the fuel tank and can then be circulated through
the fuel system. A small quantity of water will flow with the gasoline
through the carburetor metering jets and will not be especially harmful.
An excessive amount of water will displace the fuel passing through the
jets and restrict the flow of fuel; it will cause loss of power and can
result in engine stoppage.
Under certain conditions of temperature and humidity, condensation of
moisture (from the air) occurs on the inner surfaces of the fuel tanks.
Since this condensation occurs on the portion of the tank above the fuel
level, it is obvious that the practice of servicing an airplane immediately
after flight will do much to minimize this hazard.
Fuel Identification
| Gasolines containing TEL must be colored to conform with
the law. In addition, gasoline may be colored for purposes of identification.
For example, grade 100 low lead aviation gasoline is blue, grade 100 is
green and grade 80 is red. See figure 4-2.
100/130 gasoline is manufactured (1975) in two grades - high lead, up
to 4.6 milliliters of lead per gallon and low lead, not over 2.0 milliliters
per gallon. The purpose being to eliminate two grades of lower octane fuel
(80/87) and 91/96). The high lead will continue to be colored green whereas
the low lead will be blue.
The low lead will replace the 80/87 and 91/96 octane fuels as they are
phased out. Engine manufacturers have prepared instructions to be followed
in making adjustments necessary for changeover to the 100 octane fuel. |
|
A change in color of an aviation gasoline usually indicates contamination
with another product or a loss of fuel quality. A color change can also
be caused by a chemical reaction that has weakened the lighter dye component.
This color change in itself may not affect the quality of the fuel.
A color change can also be caused by the preservative in a new hose.
Grade 115/145 gasoline that has been trapped for a short period of time
in new hose may appear green. Flushing a small amount of gasoline through
the hose usually removes all traces of color change.
Fuel Identification Markings
The most positive method of identifying the type and grade of fuel includes
the following:
1. Marking of Hose. A color band not less than one foot wide painted
adjacent to the fitting on each end of hose used to dispense fuel. The
bands completely encircle the hose, and the name and grade of the product
is stenciled longitudinally in one inch letters of a contrasting color
over the color band.
2. Marking of Fuel Carriers, Pits and Fill Stands. Tags identifying
the name and grade of the product permanently affixed to each discharge
meter and fill pipe. Porcelain tags (4" x 6") carrying the same information
permanently bolted to the outside of the rear compartment of fuel servicing
equipment. The delivery pipes of truck fill stands are banded with colors
corresponding to that used on the dispensing hose.

