Parco Lubrizing
Parco Lubrizing is a chemical treatment for iron and steel parts which converts the surface to a nonmetallic oil absorptive phosphate coating. It is designed primarily to reduce wear on moving parts.
The process is a modification of Parkerizing, and consists of a precleaning treatment in which vapor degreasing, acid pickle, or spray emulsion is used, followed by a 15 minute dip in a solution of water and 10 percent by volume of Parco Lubrite. This is followed by a water rinse and a dip in water soluble oil. The phosphate surface soaks up oil and retains it.
Anodizing
Anodizing is the most common surface treatment of nonclad aluminum alloy surfaces. The aluminum alloy sheet or casting is the positive pole in an electrolytic bath in which chromic acid or other oxidizing agent produces an aluminum oxide film on the metal surface. Aluminum oxide is naturally protective, and anodizing merely increases the thickness and density of the natural oxide film. When this coating is damaged in service, it can only be partially restored by chemical surface treatments. Therefore, any processing of anodized surfaces, including corrosion removal, should avoid unnecessary destruction of the oxide film.
The anodized coating provides excellent resistance to corrosion. The coating is soft and easily scratched, making it necessary to use extreme caution when handling it prior to coating it with primer.
Aluminum wool, nylon webbing impregnated with aluminum oxide abrasive or fiber bristle brushes are the approved tools for cleaning anodized surfaces. The use of steel wool, steel wire brushes, or harsh abrasive materials on any aluminum surfaces is prohibited. Producing a buffed or wire brush finish by any means is also prohibited. Otherwise, anodized surfaces are treated in much the same manner as other aluminum finishes.
In addition to its corrosion resistant qualities, the anodic coating is also an excellent bond for paint. In most cases parts are primed and painted as soon as possible after anodizing. The anodic coating is a poor conductor of electricity; therefore, if parts require bonding, the coating is removed where the bonding wire is to be attached. Alclad surfaces that are to be left unpainted require no anodic treatment; however, if the alclad surface is to be painted, it is usually anodized to provide a bond for the paint.
Alodizing
Alodizing is a simple chemical treatment for all aluminum alloys to increase their corrosion resistance and to improve their paint bonding qualities. Because of its simplicity, it is rapidly replacing anodizing in aircraft work.
The process consists of precleaning with an acidic or alkaline metal cleaner that is applied by either dipping or spraying. The parts are then rinsed with fresh water under pressure for 10 to 15 seconds. After thorough rinsing, alodine is applied by dipping, spraying, or brushing. A thin, hard coating results which ranges in color from light, bluish green with a slight iridescence on copper free alloys to an olive green on copper bearing alloys. The alodine is first rinsed with clear, cold or warm water for a period of 15 to 30 seconds. An additional 10 to 15 second rinse is then given in a Deoxylyte bath. This bath is to counteract alkaline material and to make the alodyzed aluminum surface slightly acid on drying.
Chemical Surface Treatment and Inhibitors
As previously described, aluminum and magnesium alloys in particular are protected originally by a variety of surface treatments. Steels may have been Parco Lubrized or otherwise oxidized on the surface during manufacture. Most of these coatings can only be restored by processes which are completely impractical in the field. But, corroded areas where such protective films have been destroyed require some type of treatment prior to refinishing. The following inhibiting materials are particularly effective in the field treatment of aluminum, are beneficial to bare magnesium, and are of some value even on bare steel parts.
The labels on the containers of surface treatment chemicals will provide warnings if a material is toxic or flammable. However, the label might not be large enough to accommodate a list of all the possible hazards which may ensue if the materials are mixed with incompatible substances. For example, some chemicals used in surface treatments will react violently if inadvertently mixed with paint thinners. Chemical surface treatment materials must be handled with extreme care and mixed exactly according to directions.
Chromic Acid Inhibitor
A 10 percent solution by weight of chromic acid, activated by a small amount of sulfuric acid, is particularly effective in treating exposed or corroded aluminum surfaces. It may also be used to treat corroded magnesium.
This treatment tends to restore the protective oxide coating on the metal surface. Such treatment must be followed by regular paint finishes as soon as practicable, and never later than the same day as the latest chromic acid treatment. Chromium trioxide flake is a powerful oxidizing agent and a fairly strong acid. It must be stored away from organic solvents and other combustibles. Wiping cloths used in chromic acid pickup should either be rinsed thoroughly after use or disposed of.
Sodium Dichromate Solution
A less active chemical mixture for surface treatment of aluminum is a solution of sodium dichromate and chromic acid. Entrapped solutions of this mixture are less likely to corrode metal surfaces than chromic acid inhibitor solutions.
Chemical Surface Treatments
Several commercial, activated chromate acid mixtures are available under
Specification MIL-C-5541 for field treatment of damaged or corroded aluminum
surfaces. Precautions should be taken to make sure that sponges or cloths
used are thoroughly rinsed to avoid a possible fire hazard after drying.
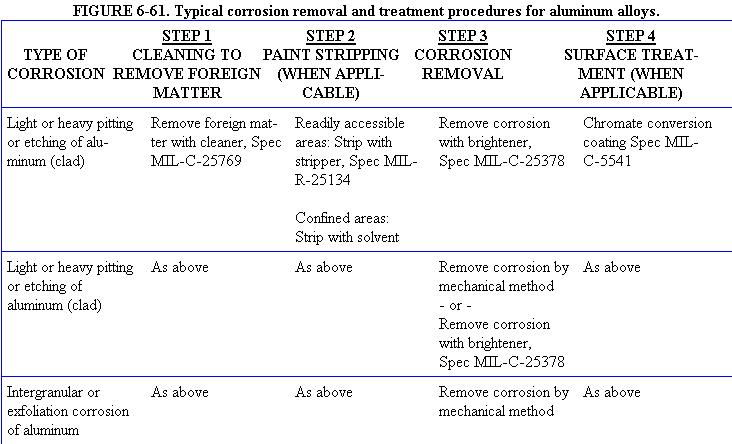
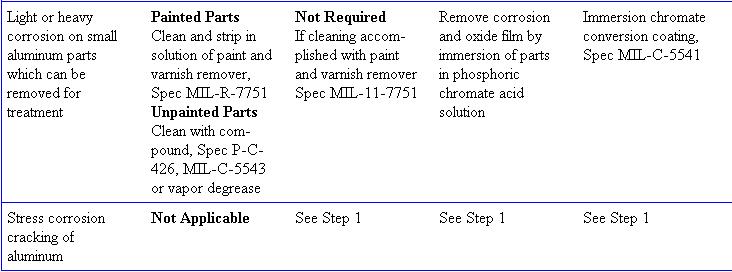
(See figure 6-61)
 |
 |