MATTER2
MATTER
| Matter can be defined as anything that has mass (weight)
and occupies space. Thus, matter is everything that exists. It may exist
in the form of solids, liquids, or gases. The smallest particle of matter
in any state or form, that still possesses its identity, is called a molecule.
Substances composed of only one type of atom are called elements. But
most substances occur in nature as compounds, that is, combinations of
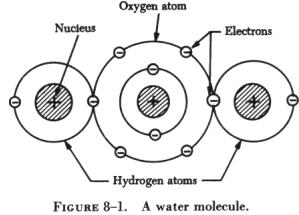
two or more types of atoms. Water, for example is a compound of two atoms
of hydrogen and one atom of oxygen. A molecule of water is illustrated
in figure 8-1. It would no longer retain the characteristics of water if
it was compounded of one atom of hydrogen and two atoms of oxygen. |
|
 |
The Atom
The atom is considered the basic building block of all matter. It is
the smallest possible particle that an element can be divided into and
still retain its chemical properties. In its simplest form, it consists
of one or more electrons orbiting at a high rate of speed around a center,
or nucleus, made up of one or more protons, and, in most atoms, one or
more neutrons as well. Since an atom is so small that some 200,000 could
be placed side by side in a line 1 inch long, it cannot be seen, of course.
Nevertheless, a great deal is known about its behavior from various tests
and experiments. |
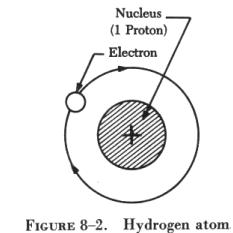
| The simplest atom is that of hydrogen, which is one electron
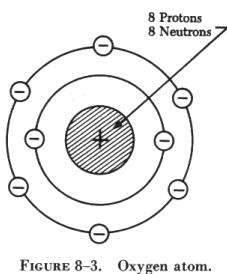
orbiting around one proton, as shown in figure 8-2. A more complex atom
is that of oxygen (see figure 8-3), which consists of eight electrons rotating
in two different orbits around a nucleus made up of eight protons and eight
neutrons.
An electron is the basic negative charge of electricity and cannot be
divided further. Some electrons are more tightly bound to the nucleus of
their atom than others and rotate in an imaginary shell or sphere closer
to the nucleus, while others are more loosely bound and orbit at a greater
distance from the nucleus. These latter electrons are called "free" electrons
because they can be freed easily from the positive attraction of the protons
in the nucleus to make up the flow of electrons in a practical electrical
circuit. |
|
The neutrons in a nucleus have no electrical charge. They are neither
positive nor negative but are equal in size and weight to the proton. Since
a proton weighs approximately 1,845 times as much as an electron, the overall
weight of an atom is determined by the number of protons and neutrons in
its nucleus. The weight of an electron is not considered in determining
the weight of an atom. Indeed, the nature of electricity cannot be defined
clearly because it is not certain whether the electron is a negative charge
with no mass (weight) or a particle of matter with a negative charge. Electricity
is best understood in terms of its behavior, which is based in part on
the charge an atom carries. When the total positive charge of the protons
in the nucleus equals the total negative charge of the electrons in orbit
around the nucleus, the atom is said to have a neutral charge. If an atom
has a shortage of electrons, or negative charges, it is positively charged
and is called a positive ion. If it possesses an excess of electrons, it
is said to be negatively charged and is called a negative ion.
Electron Movement
In a state of neutral charge, an atom has one electron for each proton
in the nucleus. Thus, the number of electrons held by the atoms making
up the various elements will vary from one, in the case of hydrogen, to
92 for uranium.
The electrons revolving around a nucleus travel in orbits, sometimes
called shells or layers. Each shell can contain a certain maximum number
of electrons, and if this number is exceeded, the extra electrons will
be forced into the next higher, or outer, shell.
The shell nearest the nucleus can contain no more than two electrons.
In an atom containing more than two electrons, the excess electrons will
be located in the outer shells. The second shell can have a maximum of
eight electrons. The third shell can hold up to 18 electrons, the fourth
32, etc. It should be noted, however, that in some large complex atoms
electrons may be arranged in outer shells before some inner shells are
filled.


