The term "pressure" as used throughout this chapter is defined as a force per unit area. Pressure is usually measured in psi (pounds per square inch). Sometimes pressure is measured in inches of mercury or, for very low pressure, inches of water.

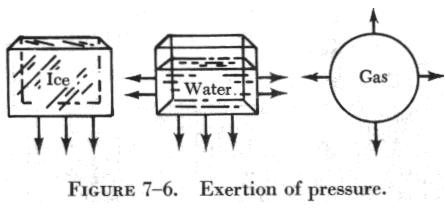
Pressure may be in one direction, several directions, or in all directions. (See figure 7-6.) Ice (a solid) exerts pressure downward only. Water (a fluid) exerts pressure on all surfaces with which it comes in contact. Gas (a fluid) exerts pressure in all directions because it completely fills the container.
Atmospheric Pressure
The atmosphere is the whole mass of air surrounding the earth. Although it extends upward for about 500 miles, the section which is of primary interest is that portion of the air which rests on the earth's surface and extends upward for about 7 1/2 miles. This layer is called the troposphere, and the higher above the earth, the lower its pressure.
This is because air has weight. If a column of air 1 inch square extending all the way to the top of the atmosphere could be weighed, it would weigh approximately 14.7 pounds at sea level. Thus, atmospheric pressure at sea level is approximately 14.7 psi. As altitude increases, the atmospheric pressure decreases approximately 1.0 psi for every 2,343 feet. However, below sea level, atmospheric pressure increases. Pressures under water differ from those under air only because the weight of the water must be added to the weight of the air.
Atmospheric pressure, its temperature effects, and the means used to measure it are discussed in greater detail in another section of this chapter.
Absolute Pressure
As stated previously, absolute temperature is used in the calculation of changes in the state of gas. It is also necessary to use absolute pressure for these calculations. Absolute pressure is measured from absolute zero pressure rather than from normal or atmospheric pressure (approximately 14.7 psi). Gauge pressure is used on all ordinary gauges, and indicates pressure in excess of atmospheric. Therefore, absolute pressure is equal to atmospheric pressure plus gauge pressure. For example, 100 psig (pounds per square inch gauge) equals 100 psi plus 14.7 psi or 114.7 psia (pounds per square inch absolute).
Incompressibility and Expansion of Liquids
Liquids can be compressed only slightly; that is, the reduction of the volume which they occupy, even under extreme pressure, is very small. If a pressure of 100 psi is applied to a body of water, the volume will decrease only 3/10,000 of its original volume. It would take a force of 64,000 psi to reduce its volume 10 percent. Since other liquids behave in about the same manner, liquids are usually considered incompressible.
In some applications of hydraulics where extremely close tolerances are required, the compressibility of liquids must be considered in the design of the system. In this study, however, liquids are considered to be incompressible.
Liquids usually expand when heated. This action is normally referred to as thermal expansion. All liquids do not expand the same amount for a certain increase in temperature. If two flasks are placed in a heated vessel, and if one of these flasks is filled with water and the other with alcohol, it will be found that the alcohol expands much more than the water for the same rise in temperature. Most oils expand more than water. Aircraft hydraulic systems contain provisions for compensating for this increase of volume in order to prevent breakage of equipment.
Compressibility and Expansion of Gases
The two major differences between gases and liquids are their compressibility
and expansion characteristics. Although liquids are practically incompressible,
gases are highly compressible. Gases completely fill any closed vessel
in which they are contained, but liquids fill a container only to the extent
of their normal volume.