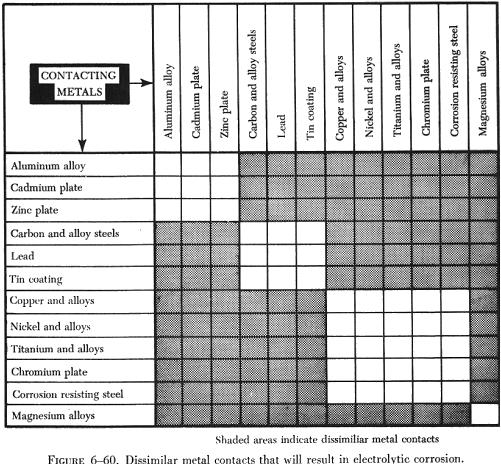
Certain metals are subject to corrosion when placed in contact with other metals. This is commonly referred to as electrolytic or dissimilar metals corrosion. Contact of different bare metals creates an electrolytic action when moisture is present. If this moisture is salt water, the electrolytic action is accelerated. The result of dissimilar metal contact is oxidation (decomposition) of one or both metals. The chart shown in figure 6-60 lists the metal combinations requiring a protective separator. The separating materials may be metal primer, aluminum tape, washers, grease, or sealant, depending on the metals involved.

Contacts Not Involving Magnesium
All dissimilar joints not involving magnesium are protected by the application of a minimum of two coats of zinc chromate primer in addition to normal primer requirements. Primer is applied by brush or spray and allowed to air dry 6 hours between coats.
Contacts Involving Magnesium
To prevent corrosion between dissimilar metal joints in which magnesium alloy is involved, each surface is insulated as follows:
At least two coats of zinc chromate are applied to each surface. Next, a layer of pressure sensitive vinyl tape 0.003 inch thick is applied smoothly and firmly enough to prevent air bubbles and wrinkles. To avoid creep back, the tape is not stretched during application. When the thickness of the tape interferes with the assembly of parts, where relative motion exists between parts, or when service temperatures above 250° F are anticipated, the use of tape is eliminated and extra coats (minimum of three) of primer are applied.